
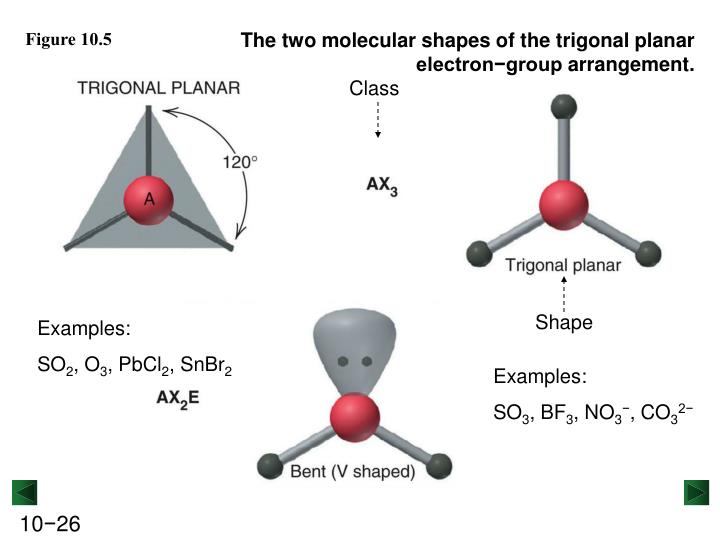
Note that the alternative Lewis structure #O=S=O# is still bent (why?), and entirely consistent with the structural parameters I have advanced. The longer #S-O# bonds, and the more diffuse sulfur lone pair, tend to diminish the repulsive properties of the the sulfur lone pair. Sulfur desires 6 electrons, and so does oxygen. SO2 is a bent shape (molecular geometry).
The hybridization of the two oxygen atoms is sp2 as properly.

The actual which is only slightly compressed from the idealized trigonal planar geometry. A description of the hybridization of SO2 including sigma and pi bonds.Note that the SO2 hybridization is sp2 for the central sulfur atom. Of course, the 2 oxygen atoms are entirely equivalent, and the resonance isomerism available to #O=S^(+)-O^-# does reflect this. If you want to keep sulfur with an octet, it still has a. Molecule / (1) SO2 / (2) HBF2 / (3) XeF4 / (4) CH2Cl2 / (5) NF3 (a) No. SO2 is bent (C2v) because sulfurs VSEPR geometry is trigonal planar the third group is the lone pair. Then, identify the correct the molecular shape and bond angle. Refer to your textbook for a more thorough discussion. Introduction: (Below is a review of concepts that you should have learned in your lectures. Certain molecular models will be provided and the molecular geometry and polarity are to be determined also. The molecule is polar The electron pair geometry around the central atom is trigonal planer. Since for a Group 16 atom, there should be 6 valence electrons for neutrality, the assigned electronic charges are consistent with the Lewis representation. For each of the following molecules, draw the Lewis Diagram and tally up the electron pairs. molecular geometry is to be named, and its molecular polarity is to be determined. See here theyre given actually sulfur dioxide S. The SO2 bond angle will be 120 degrees since it has a Bent molecular geometry. The lone pair of electrons is at the top of the SO2 molecule. When they do so they are forced to the opposite side of the Sulfur atom giving SO2 a Bent molecular shape. Count the total number of electron domains around the central atom. This is because the Valence Shell Electron Pairs Repel each other. We use the electron domain geometry to help us predict the molecular geometry. To determine the shape of a molecule we distinguish between lone pairs and bonding pairs. It is important to note that electron-pair geometry around a central atom is not the same thing as its molecular. There are 18 electrons to distribute in the molecule, where sulfur, as the LEAST electronegative atom, will be central.Ī Lewis structure of #O=S^(+)-O^-# is reasonable, where from left to right as we face it, there are 6, 5, and 7 valence electrons. The molecular geometry is the arrangement of the atoms in space. Although this may be confusing since there are different bonds, a double bond and a single bond is present, including triple bonds of the molecule, it still comes up with the same bond angles on all.#"VSEPR"# predicts that sulfur dioxide should be a bent molecule, where, to a first approximation, Why? This means that the o-orbitals are also almost perfect SP2 orbitals. Hi Guys Today in this video we are going to share a step-by-step procedure to determine the molecular geometry of SO2 molecules. Looking at the molecular geometry of SO2, the bond angle is at 120°. The CO2molecule does not have a netdipole due to its linear geometry. There is polarization because Sulfur pulls the charge of the molecule to its side while gaining partial negative charge and not Oxygen since it is the least electronegative atom, making SO2 a polar molecule. CO2 and SO2 based on their molecular from CHM 151 at Rio Salado Community. There is an imbalance charge across other atoms in the molecule, making Sulfur Dioxide polar. With SO2, the pairs of bonding electrons are arranged at an angle of 120 degrees. Consequently, 6×three18 valence electrons distribute in the course of the structure placing 4 for every of two double bonds makes use of up to eight. Electron geometry includes all the pairs of electrons, even the lone pairs. A quick explanation of the molecular geometry of SO2 including a description of the SO2 bond angles.We can see that there are only two atoms attached to the. The hybridization of the two oxygen atoms is sp2 as properly. This is not similar to molecular geometry, where only the total number of atoms is considered to determine its shape.

The electron geometry of SO2 is trigonal planar.


 0 kommentar(er)
0 kommentar(er)
